 |
MicroPseudo | MicroReference not available |
MicroReference Refillable |
|---|---|---|---|
| Setup? | 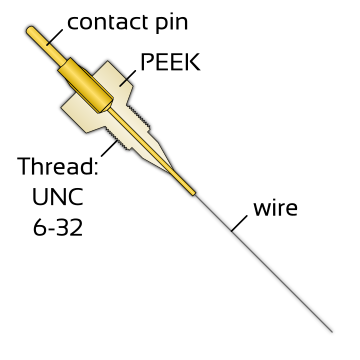 |
 |
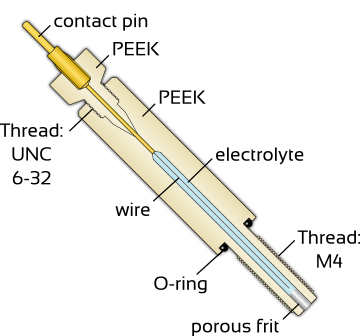 |
| Electrode? | Pt / Ag / custom | Ag / custom | Pt / Ag / custom |
| Electrolyte? |  |
 |
fillable by customer |
| Electrolyte composition? |  |
|
fillable by customer |
| Refillable? |  |
 |
 |
| Tip diameter? | 0.5 mm | 0.8 mm | 4 mm |
Please note:
Electrochemical experiments can be performed using either a 2-, 3- or 4-electrode setup. Except for the case of a 2-electrode configuration at least one (in the ideal case currentless) reference electrode is required. However, a miniaturization of the measuring cells is considerably demanding for the design of the reference electrode. In case of nonaqueous systems, there are even further challenges.
rhd instruments offers a number of different (non)aqueous micro-reference electrodes based on capillaries encased by PEEK or polyimide. Amongst others, they are available for:
- aqueous solutions
- ionic liquids
- DMSO based electrolytes
- organic carbonates (e.g. battery electrolytes)
We are also happy to develop an individual reference electrode for your system together with you - please feel free to contact us.
All these reference electrodes are delivered with a suitable storage container filled with the internal electrolyte of the reference electrode. It is suitable for regeneration after cleaning as well as for long-term storage.
If appropriate handled and properly stored potential stability of at least 2 months should be given. In case of nonaqueous micro-reference electrodes we recommend a calibration of the potential to the half-wave potential of the ferrocene redox peaks.
Besides "real" reference electrodes we also offer you so called pseudo-reference electrodes consisting of silver or platinum wire. The following table gives an overview of available materials and the compatibility with different cell types.
- Impedance spectroscopy studies of the structure and dynamics of the electrochemical double layer between metal electrodes and ionic liquids with potential and temperature control
- Determination of the stability window of ionic liquids and carbonate based battery electrolytes using cyclic voltammetry
- Cyclic voltammetry studies of solutions of organic dyes in acetonitrile and dichloromethane for determination of the HOMO-LUMO gap







 Add to Request
Add to Request



